Abstract
Background: Peripheral T-cell lymphoma (PTCL) is a family of aggressive lymphomas. Standard therapies in the relapsed or refractory (R/R) setting provide single-agent overall response rates (ORR) of <30%. Duvelisib (DUV), an oral inhibitor of phosphatidylinositol 3-kinase (PI3K)-δ and PI3K-γ isoforms, is being investigated for R/R PTCL in the Phase 2 PRIMO trial (NCT03372057; supported by Secura Bio®). To date, this trial of DUV has demonstrated an ORR ~50%. We present preliminary outcome data by prior treatments and updated safety analyses of the PRIMO Expansion Phase (EP); the trial is fully enrolled as of January 2022.
Methods: Eligibility criteria include adults with pathologically confirmed PTCL (WHO criteria) after ≥2 cycles of ≥1 prior standard regimen, and a CD4 lymphocyte count of ≥50/mm3. Based on dose optimization results, the EP dose is DUV 75mg BID for 2 cycles, to maximize disease control, followed by 25mg BID, to mitigate late toxicities, until progressive disease (PD) or unacceptable toxicity. Prophylaxis is required for PJP and recommended for herpes simplex/varicella zoster. Primary endpoint is ORR by IRC using Lugano 2014 criteria evaluated in all patients (pts) who received at least 1 dose of DUV.
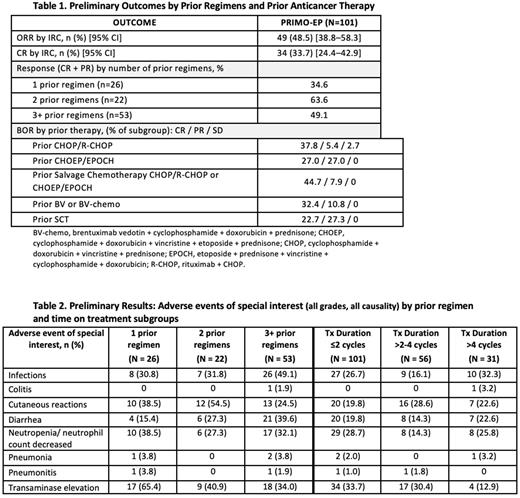
Results: Updated analyses of the PRIMO EP included 101 pts of 125 planned (data cut-off 10/1/21) with a median follow-up of 8.7 months from time of first response. Median age was 67.0 years (range 21-92) with a median 3 prior lines of therapy (range 1-9). Prior treatments received included CHOP/R-CHOP, CHOEP/EPOCH, or BV/BV-chemo (all 36.6 %), salvage chemo after CHOP/R-CHOP or CHOEP/EPOCH (37.6%), and SCT (21.8%). Mean treatment duration was 17.1 weeks. Median (range) treatment duration was 9.0 (1-79) weeks. Exposure by treatment duration included 43.6% (≤2 cycles), 23.8% (>2-4 cycles), and 30.7% (>4 cycles). The ORR by IRC was 49% with a CR rate of 34%. In pts with ≥3 prior lines, the ORR was maintained (49.1%). BOR (Best Overall Response by Lugano classification) by prior regimens and prior anticancer therapy are shown in Table 1. Median (range) PFS was 3.62 (0.03+ -17.2+) months. Median duration of response was 7.7 months, and 7.4 months for those in CR. TEAEs leading to dose hold or dose reduction occurred in 37.6% and 3.0% of pts. Adverse events (AEs) (all causality) occurring in >5% of pts with maximum GR3 were alanine aminotransferase/aspartate aminotransferase (ALT/AST) increased 14.9%/13.4%, rash maculo-papular and diarrhea (both 7.9%) neutropenia (13.8%), and with a maximum GR4 was ALT increased (5.9%). Adverse events of special interest (AESI) included infections, colitis, cutaneous reactions, neutropenia, diarrhea, pneumonitis, and transaminase elevation. Incidence of transaminase elevation was less at Cycle >4 (12.9%), versus at Cycle ≤2 (33.7%). Incidence of pneumonia, pneumonitis, and colitis was low regardless of treatment duration (Cycle ≤2 to Cycle >4: range 0-3.2%), and the rates of some AESIs increased slightly from Cycle ≤2 to Cycle >4 (infections increased by 5.6%, diarrhea and cutaneous reactions both increased by 2.8%). Transaminase elevations decreased, and diarrhea and infections increased as number of prior anticancer regimens increased. TEAEs resulting in death (other than PD) were seen in 8 pts; 1 patient each experiencing GI hemorrhage, vascular dementia, acute cholecystitis, hypoxia, suicide (unlikely/unrelated), and sepsis, EBV-associated lymphoproliferative disorder, and pneumonitis (treatment-related). AESIs by select subgroups: See Table 2.
Conclusions: In this updated, expanded analysis of the PRIMO study, response by type of prior anticancer therapy was generally consistent with the overall population (range 43.2% to 54%). Response rates ≥35% were seen regardless of number of prior regimens. The types of AEs seen were consistent with those observed previously in the PRIMO trial with no additional unexpected treatment-related toxicities. Incidence of infections increased with number of prior therapies; transaminase elevations decreased as treatment duration increased. These preliminary data show promising results for duvelisib warranting further research in a disease set with high unmet needs, poor prognoses, and limited effective treatment options. DUV is now listed on the National Comprehensive Cancer Network® T-cell Lymphoma Guidelines® as a Category 2A other recommended regimen for R/R PTCL (all subtypes).
Disclosures
Zinzani:Secura Bio: Membership on an entity's Board of Directors or advisory committees; Sandoz: Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Zain:Secura Bio: Consultancy, Research Funding; Daichi Sankyo: Consultancy, Research Funding; AstraZeneca: Research Funding; Myeloid: Consultancy, Research Funding; CRSPR: Research Funding; Seattle Genetics: Research Funding, Speakers Bureau; 3M: Current holder of stock options in a privately-held company; Affirmed: Research Funding; Kiyowa Kirin: Speakers Bureau. Casulo:Bristol Myers Squibb: Research Funding; Genentech: Research Funding; Gilead: Research Funding; Verastem: Research Funding; Secura Bio: Research Funding. Gritti:Kite-Gilead: Other: Advisory Board (2020); Incyte: Other: Training activity (2022); Takeda: Other: Advisory Board (2020, 2021, 2022), Training activity (2020, 2022), Individual scientific consultancy (2021-ongoing); Clinigen: Other: Training activity (2021); Sandoz: Other: Support for attending meetings (2021); Italfarmaco: Other: Advisory Board (2021); Beigene: Other: Training activity (2022); Ideogen: Other: Advisory Board (2022), Training activity (2022); Roche: Other: Advisory Board (2021) Training activity (2020), Support for attending meetings (2021); Genmab: Other: Advisory Board (2022); IQVIA: Other: Advisory Board (2020). Izutsu:Yakult: Research Funding; Beigene: Consultancy, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Ono Pharmaceutical: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Kyowa Kirin: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Chugai: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Janssen: Research Funding; Merck Sharp & Dohme: Honoraria, Research Funding; Genmab: Research Funding; Loxo Oncology: Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Symbio: Honoraria; Eli Lilly and Company: Consultancy, Honoraria; Eizai: Honoraria, Research Funding; Incyte: Research Funding. Brammer:DrenBio: Consultancy; Seattle Genetics: Speakers Bureau; Kymera Therapeutics: Consultancy; Bristol-Myers Squibb: Research Funding. Mehta-Shah:Verastem: Research Funding; Secura Bio: Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kyowa Hakko Kirin Co., Ltd.: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genetech/Roch: Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees, Research Funding; Corvus Pharmaceuticals: Research Funding; Celgene: Research Funding; Bristol Myers-Squibb: Research Funding. Pro:Seattle Genetics: Honoraria. Horwitz:C4: Research Funding; Yingli Pharma Limited and Tubulis: Honoraria; Cimieo Therapeutics: Honoraria; Shoreline Biosciences, Inc.: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Affimed,: Consultancy; Kyowa Hakko Kirin: Research Funding; Crispr Therapeutics: Research Funding; Celgene: Research Funding; Seattle Genetics,: Research Funding; Kyowa Hakko Kirin: Consultancy; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; ONO Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; SecuraBio: Honoraria; Millennium /Takeda: Research Funding; Daiichi Sankyo: Research Funding; ADC Therapeutics: Research Funding; Affimed: Research Funding; Verastem/SecuraBio: Research Funding.
OffLabel Disclosure:
Duvelisib, an oral inhibitor of phosphatidylinositol 3-kinase (PI3K)-ÃŽÃ'´ and PI3K-ÃŽÃ'³ isoforms, is being investigated for use in peripheral T-cell lymphoma (PTCL).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal